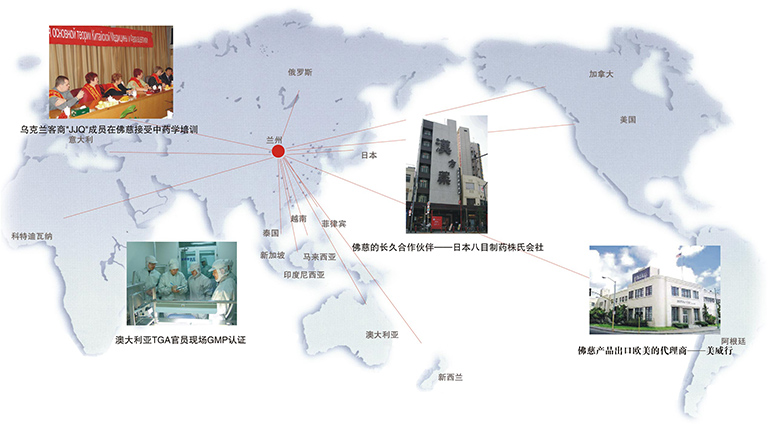
In 1931, Lanzhou Foci’s Min Shan Brand products became the first traditional Chinese medicines to be sold internationally. Its first international markets were Southeast Asia and Japan, and were welcomed by overseas Chinese who recognized the quality and efficacy of the brand.
The popularity of Min Shan Brand gained an even wider audience after receiving GMP certification from the Australia’s Therapeutic Goods Administration (TGA) in 1996, China’s State Food and Drug Administration (SFDA) in 1999, and Japan’s Ministry of Health, Labor, and Welfare (MHLW) in 2006. The over 300 products, including pills, tablets, capsules, and granules under the Min Shan and Foci Brand names are trusted and welcomed by practitioners and consumers around the world. Min Shan Brand is now exported to over twenty-seven countries and areas including the United States, Canada, Japan, Singapore, Indonesia, Thailand, Malaysia, New Zealand, Australia, Russia, Ukraine and Hong Kong.